Research
Our group develops next generation optical imaging and sensing platforms in the context of future biomedical, pharmaceutical and clinical applications. We are particularly interested in label-free or digital approaches: low-cost key-enablers for democratizing state-of-the-art healthcare globally. We realise this vision by combining fundamental research with systematic platform and assay development. Sometimes, we meet our target by simplifying and adopting existing technologies. On many occasions, however, we start from a curiosity-driven hypothesis where applicability is a long term high-gain objective. To realise our vision, we combine an interdisciplinary toolbox ranging from molecular biology over ultrafast optics to digital holography with the key-ingredient being creativity and room for personal development. Selected research topics are outlined below.
Phototransient holography
Phototransient holography is a technique that we pioneered over the past few years: It enables high-speed widefield observations of transient dynamics and photoinduced signals on conventional cameras. We use light to heat a material and then detect transient phase-changes, on femtosecond to picosecond time-scales. Our approach has several key-advantages over alternative techniques: its transient, rather than steady-state, read-out enables large field-of-view observations at dramatically improved signal-levels.
Over the past few years we have done most of the necessary proof-of-concept experiments. We implemented holographic all-optical lock-in detection and demonstrated both spectroscopic and sensing applications. We, furthermore, performed phototransient 3D particle-tracking experiments and recorded time-resolved transients on freely moving samples.
The current focus is on adopting the technique to biomedical imaging and identification of bacteria, cells and tissue. As such, we are implementing a system that combines high-intensity infrared (IR) pulses with visible, holographic, read-out of the phototransient signal. This combination allows recording vibrational, or chemical, fingerprints of biologically and clinically relevant samples.
Liebel et al. Nanoscale 14, 3062 (2022); Block et al. Nat. Nanotechnol. 16, 1195 (2021); Liebel et al. Nano Lett. 21, 1666 (2021); Block et al. Sci. Adv. 5, eaav8965 (2019)
Quantitative mass imaging
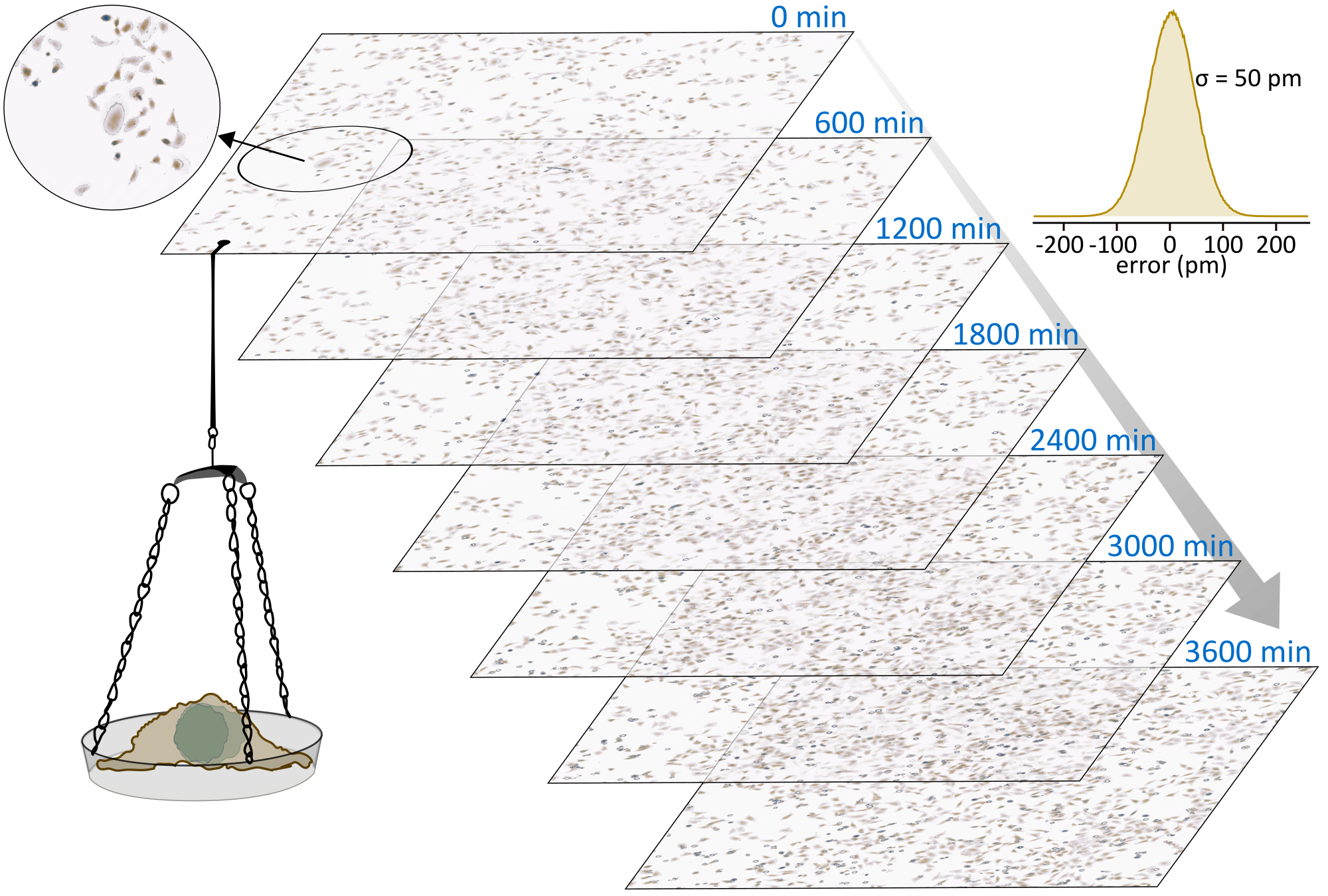
Quantitative mass imaging relies on the linear relationship between phase-retardation and biological mass of a sample. In other words: it is possible to shine light through, for example, a living cell to directly determine its weight in an all-optical, label-free manner. All that is necessary is a quantitative phase measurement of the transmitted wavefront. Quantitative mass imaging has an incredible potential for both fundamental biological as well as clinical applications. To deliver this promise very accurate and precise mass imaging platforms are necessary that are able to time-lapse interrogate large sample areas over hours to days while maintaining physiologically relevant conditions.
To ensure a seamless integration into analytical workflows we are currently working on the details: Our focus on establishing a robust and easy-to-use spatially and temporally incoherent light-based quantitative mass imaging platform: We want ultimate mass-sensitivity without compromise. At our current sensitivity-limit of around 50 pm, or half the diameter of a Hydrogen atom, we are starting to be able to compete with very complex, and low-throughput, systems such as microchannel resonators or cantilever-based measurements. Most importantly, the all-optical nature of quantitative mass imaging enables easy-to-analyse assumption-free measurements over gigantic observation areas exceeding 10x10 mm.
Liebel et al. Sci. Adv. 6, eabc2508 (2020); Liebel et al. Nat. Nanotechnol. 15, 1005 (2020)
Nanosizing
Nanoparticles are everywhere: proteins are natural nanoparticles, viruses try to infect us and cells use extracellular vesicles for communication. Unsurprisingly, detecting, quantifying and analysing these biomarkers is of utmost importance. Analogous to macroscopic objects, the shape and size of nanoparticles is tightly coupled to their function. Precisely and accurately measuring these parameters on the nanoscale is crucially important: it allows us to develop better diagnostics or drugs, and detect pathogens and contaminations in food or water.
We develop highly sensitive and accurate all-optical tools to measure these particles. Our methods are label-free. It makes them easy and cheap to implement but such methods are often prone to false positives. We are currently addressing this challenge by simultaneously measuring multiple parameters using a single, turn-key, holographic platform. We just concluded a major proof-of-concept phase and will now be transitioning towards applications. Our target application is precise and accurate extracellular vesicles characterisation and distinguishing them from a major contaminant: protein aggregates. In parallel, we are tailoring the platform to fit into the routine clinical analysis workflow.
Ortiz-Orruño et al. ACS Nano 17, 221 (2023); Saemisch et al. Nano Lett. 21, 4021 (2021); Ortiz-Orruño et al. Nano. Lett. 21, 317 (2021); Liebel et al. Nano Lett. 17, 1277 (2017)
High throughput SERS
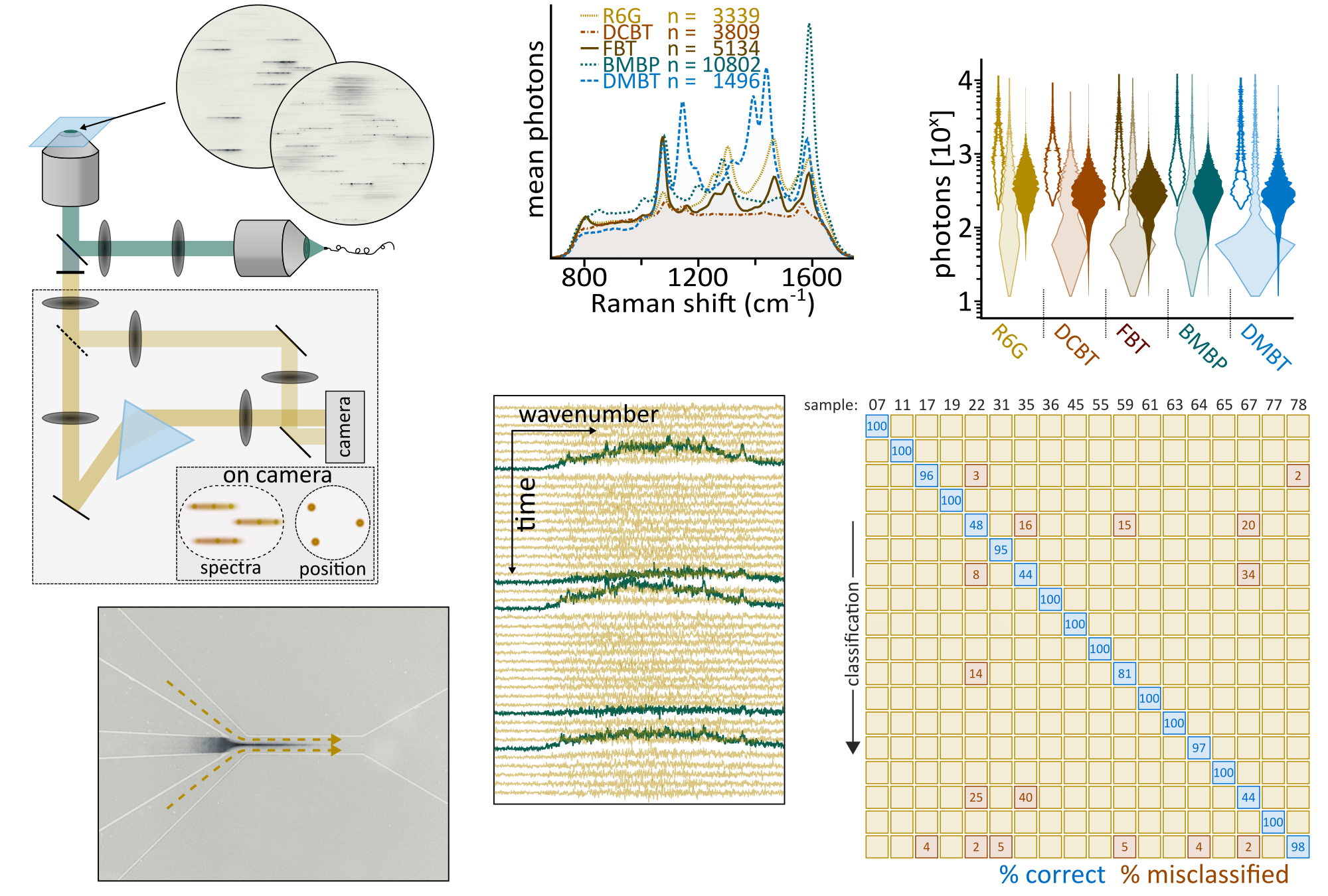
SERS-based diagnostics and sensing requires: excellent, tailor-made, SERS probes combined with spectroscopy and imaging of equal quality. Together with Ramón Álvarez Puebla’s group we aim at providing the right mix. We combine tailor-made particles and target-specific imaging solutions. The latter is the main expertise of this group. Our long-term goal is to provide analytical platforms that harness the potential of single-particle observations but nevertheless interrogate relevant sample volumes of several millilitres within a few minutes. This vision necessarily requires us to go beyond confocal Raman.
In a first step, we performed combined holographic and widefield Fourier-transform SERS imaging, an approach that allowed us to record time-lapse videos of SERS particles inside live cells. We have since then considerably improved the SERS probe-throughput towards several thousand particles within a few tens of minutes. Currently, we are improving both detection-frequency and specificity, technical aspects that allowed us to detect >1000 SERS codes in under a minute. These advances allowed us to start building large SERS code-libraries. Our highly multiplexed, yet single particle observation-based, approach enables very accurate particle-classification which will enable identifying key-biomarkers at very low concentrations within very large sample volumes.
Liebel et al. Angew Chem. Int. Ed 61, e202200072 (2022); Liebel et al. Nat. Nanotechnol. 15, 1005 (2020); Becerril-Castro et al. ACS Appl. Mater. Interfaces (2022)